What is polyurethane foam? Consumers and manufacturers alike may want to know the answer to this question. Are you a polyurethane foam technician, a plant manager, or the owner of the foaming plant itself? Do you want a stronger foundational understanding of how polyurethane flexible foaming actually works?
This article will detail the fundamental elements of polyurethane foaming, particularly as it applies to continuous flexible foaming.
At its most basic, polyurethane foam does two things in the factory. From the liquid stage it:
- expands
- and gels.
The liquid first expands as air bubbles are introduced, then a secondary reaction gels, or hardens the material at some point in that expansion.
So how does it expand and gel?
Part I: Isocyanates and Polyols
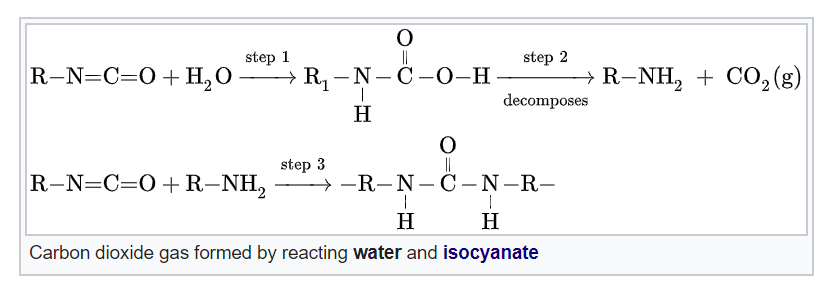
We have two foundational chemicals involved in the foaming of PU. Isocyanates, by definition, have the functional group R−N=C=O. Polyols, by definition, contain multiple hydroxyl, or −OH functional groups.
(Functional groups are basically ways to recognize huge molecules in organic chemistry. Certain combinations of certain elements react in specific and predictable ways, e.g. a molecule with an −OH group will have a high boiling point.)
In the world of foaming, the two most common isocyanates are TDI (for flexible and semi-rigid foaming) and MDI (for rigid foaming). Let’s use the shorter terms “TDI” and “polyol” for the rest of this article.
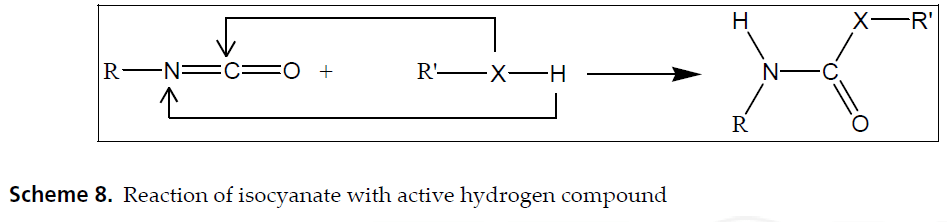
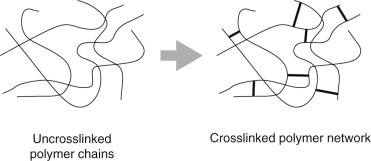
There are plenty of articles out there that break down the hard chemistry of this reaction. For our purposes, let’s just take note of the fact that when TDI and polyol react, and they both have multiple functional groups per molecule (the “DI” stands for diisocyanate, or two isocyanates, and polyol obviously contains multiple hydroxyl groups), they form branched or cross-linked polyurethane. This is the start of our polyurethane polymer.
A polymer is a big, or macromolecule composed of multiple subunits. If unit A links with another unit A links with another unit A, they make a linear, unbranched polymer. If unit A can link at once to two other unit A’s, they make branched and cross-linked structures. To compare their functional difference, just imagine climbing a rope versus climbing a ladder. The rope may be strong, but is structurally susceptible to bending and reshaping. Conversely, a ladder has multiple points of tension that allow for weight distribution. This manifests as elasticity, where the material can be twisted and stretched to certain degrees and still return to its original shape.

Alright, so TDI + polyol has made our polyurethane polymer, which now can be expanded to make foam. To do that, we add water. Isocyanate is highly reactive, and produces two things with water: urea linkages/urethane, and carbon dioxide (CO2) gas.
This is part two of our basic process: the gelling, or gellation of our material. This means the polyurethane is secured to a definite shape.
Part II: Blowing the Foam
The CO2 is considered our primary blowing agent. As a gas, it blows little air pockets into the PU to form the foam. Just like blowing soap bubbles though, there is a point when the liquid casing cannot withstand the pressure of the air inside and bursts.
Here’s where gellation comes in, and why it’s so important. To successfully make material foam, the polyurethane must gel into shape once air has been blown to it. It’s harder than it sounds, and it’s also why foam technicians can make or break your factory production. The art of foam-making revolves around this sensitive balance between expansion, or blowing, and gelling.
Once they’re formed inside the polyurethane, we’re going to call the air bubbles cells. If the foam expands and gels before any air bubbles burst, you get closed-cell foams, which are semi-rigid (not truly rigid like foam made of MDI) since the material doesn’t bend as easily. To go back to our ladder metaphor, closed-cell foams are like wood ladders, whereas open-cell foams are like rope ladders. If some or all cell walls are allowed to rupture before gellation, you get a much more flexible material that bends, twists, and even breaks easier.
So what is polyurethane foam? Basically: TDI, polyol, water, polymer, open versus closed-cell. That’s the baseline, and now we’ll introduce the additives.
Part III: Additives
Let’s break down PU foaming additives by function. One of the most important additives is the catalyst, which can affect the basic reactions in several ways. It can speed the expansion, speed the gelling, cool the reaction (so you have less of a fire hazard on your hands), etc. There are also curing agents, which include chain-extenders and cross-linking agents. Chain-extenders, like their name suggests, extend polymer chains, which increases material flexibility. Cross-linking agents promote and strengthen cross-linkages, increasing structural integrity for more rigid foams.
Think of surfactants like emulsifiers. Oil and water on their own do not mix, but once you add some dish soap, they can be emulsified into a uniform mixture. Surfactants work like the soap. A more uniform mixture means a smoother reaction, and you get more even cell sizes, steadier reaction speeds, and finer control between gellation and foam collapse.
(The reason they’re called surfactants is because they reduce the surface, or interface tensions between two compounds. As in, the oil doesn’t just sit neatly on top of the water—surfactants blend that interfacing surface between them.)
Remember that CO2 gas from the reaction with water acts as a blowing agent? Well, other blowing agents may also be used or added. The main inconvenience of water blowing is the high temperature of the reaction, making PU foaming a fire hazard. Physical blowing agents (additives that physically encourage the expansion of cells instead of that initial CO2, which is chemically blown) reduce that fire hazard.
A similar class of additives is fillers. They come as particles or fibers. Particulate fillers can reduce flammability and add weight to foam (good for cushioning foams). Fibrous fillers reinforce cell structure. All fillers function to 1) add physical properties like tensile or compressive strength to foam, and 2) save on costs by reducing the amount of liquid chemicals used per batch.

Finally, we have the additive that most people know about: flame retardants. First, to combat flammable foam products, countries added flame retardant requirements to PU production. However, several wide-used flame retardants have proven to have negative health effects on consumers, so countries then changed flame retardant regulations. As it stands, different countries have different sets of regulations on additive types, whether PU products have to pass an open-flame or a char test, etc. Different regions also have different degrees of access to types of flame retardants. We have an upcoming article that will go further into detail about this very debate, but for now, suffices to say that this is an element of foam quality that can greatly impact your consumer market.

(Final aside: a class of additives we won’t get into detail about here is colourants, because simply, they just add color to your foam.)
Part IV: For Example…
Let’s have a concrete example. Here at Sunkist, this is our prototypical flexible foaming recipe:
Isocyanate: TDI
Polyol: polyol
Blowing Agents: water, methylene-chloride (MC)
Catalysts: amine, tin
Surfactant: silicone
Now we understand what each element does during the foaming process. TDI + polyol begins the creation of polyurethane. A mixing head first injects small amounts of air into the liquid mix to kickstart the foaming process. TDI + water chemically produces the CO2 gas that blows the liquid into foam. In addition, we add MC so that less water is used in the initial reaction and the overall reaction temperature is lower, all while cell expansion is retained.
Meanwhile, the amine additive is doing multi-purpose catalysis (speeding the reaction) and tin provides a stable gelling catalyst, increasing foam structural elasticity. Silicone smooths and steadies the entire blowing process, maintaining cell structure evenness until gellation occurs.
And there we have it! Sunkist’s answer to the question “what is polyurethane foam,” laying out every basic element of the recipe. The chemist remains the true expert on ingredient quantities. However, if every foam technician, machine operator, and even factory owner has a foundational understanding of what’s actually going on in a foaming machine? Your plant will have built-in, well-informed quality-control in every step of the production process.
Want to keep up to date on our company news? Take 1 minute to fill out the form below.